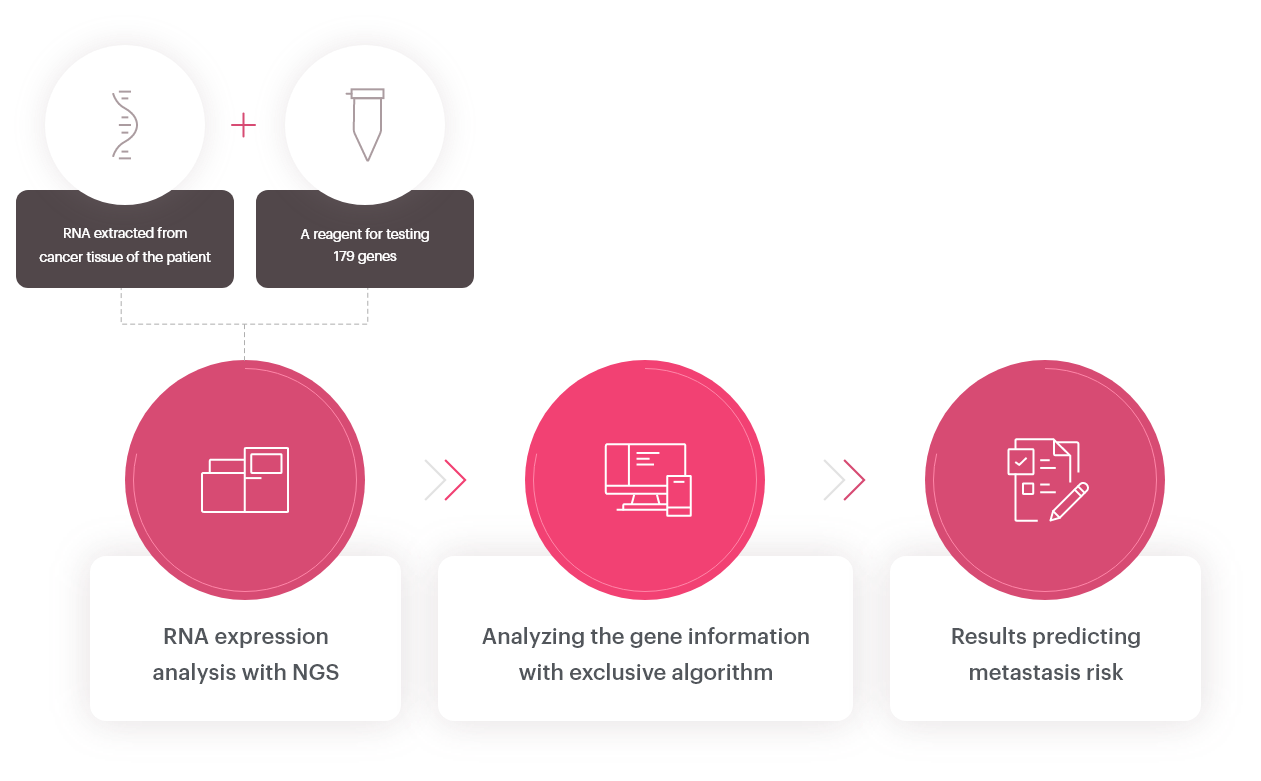
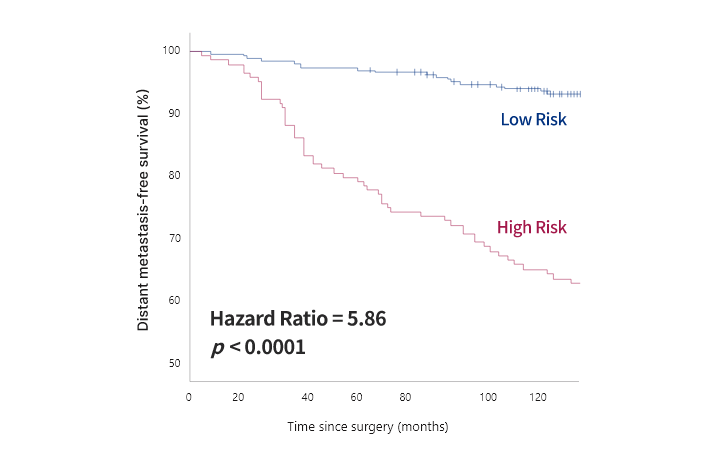
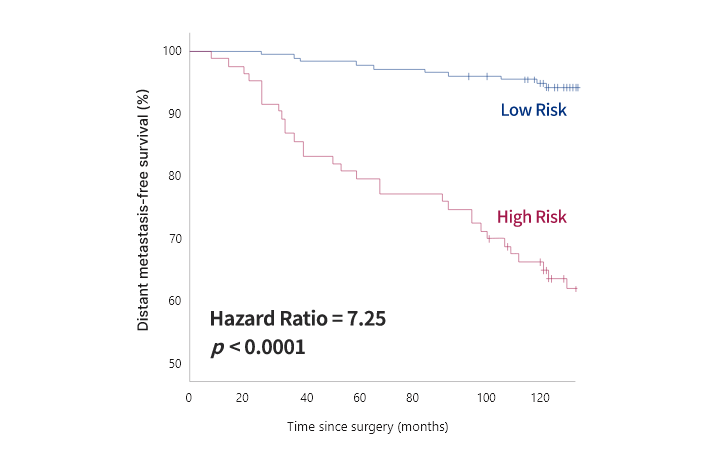
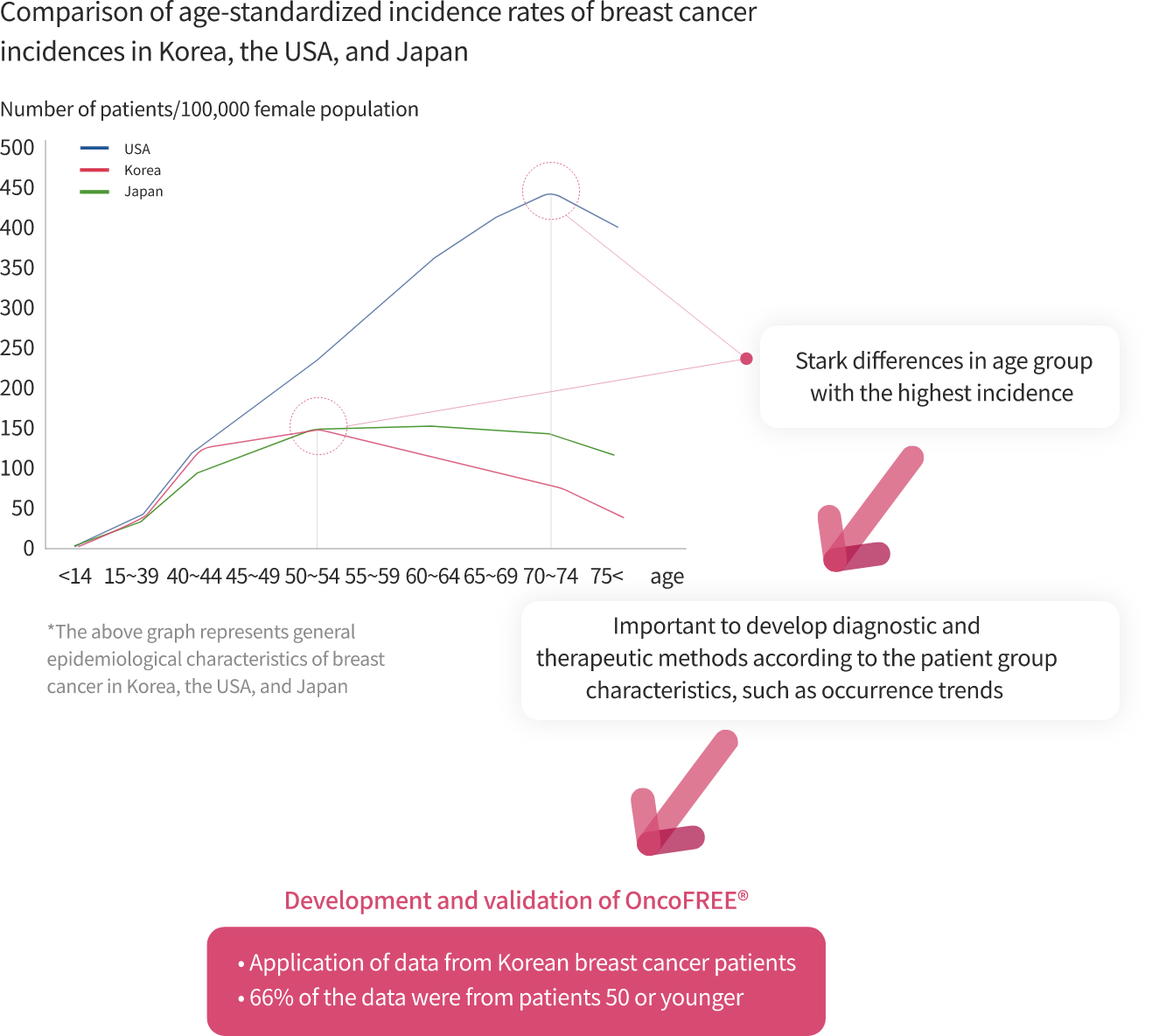
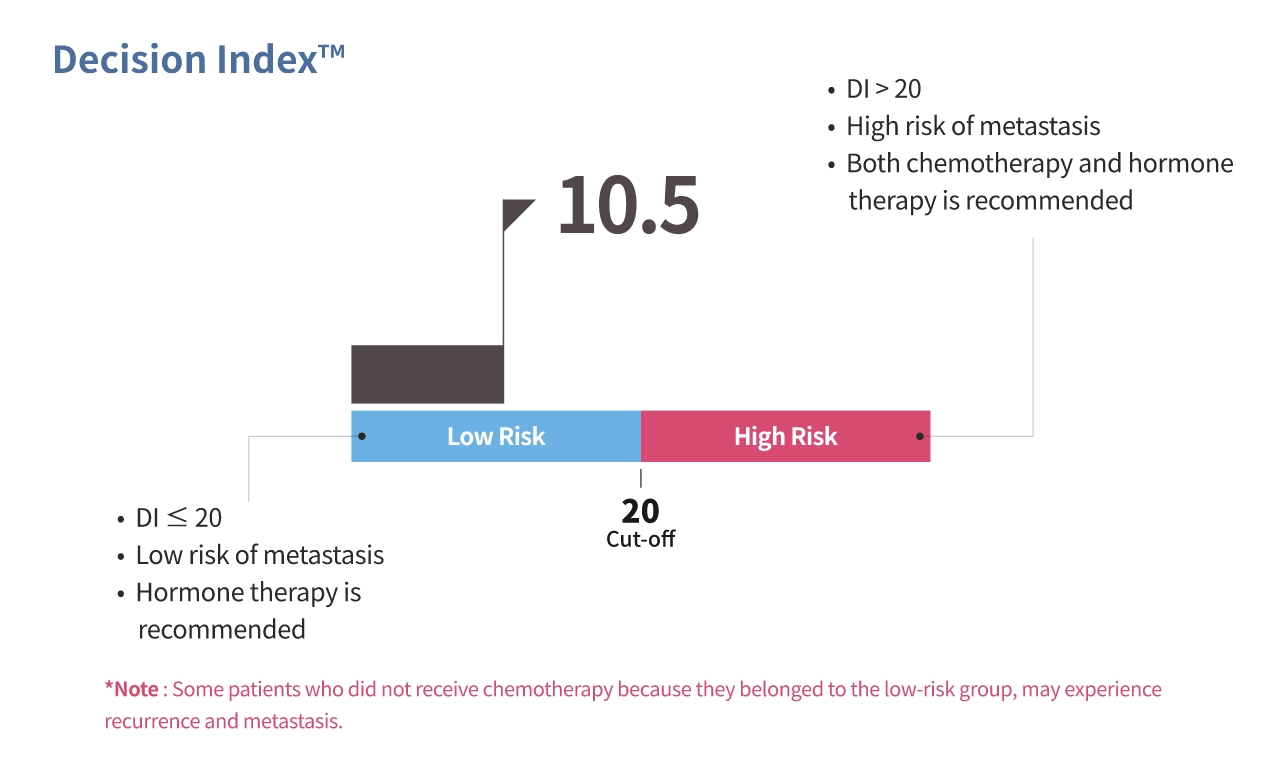
OncoFREE® is
an in vitro diagnostics multivariate index assay (IVD-MIA) which predicts prognosis
based on the risk for distant metastasis in hormone receptor-positive,
HER2-negative early-stage breast cancer patients with no axillary lymph node metastasis,
by analyzing breast cancer tissue RNA expression data.